Which of the Following Is a Buffer Solution
Buffer solutions are obtained when a weak acid is mixed with its conjugate base or a weak base is mixed with its conjugate acid. It exhibits a definite pH and it sustains it that is it prevents the change in pH or hydrogen ion change.
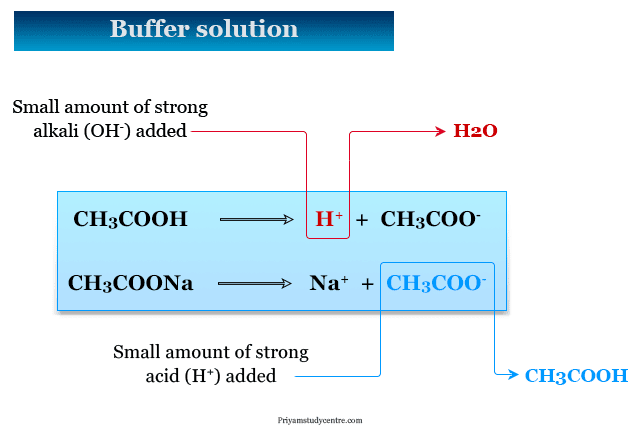
An acid buffer solution consists of solution of a weak acid and its salt with strong baseThe best known example is a mixture of solution of acetic acid and sodium acetate.

. Ka HCOOH 18 x 104. C add some NaOH into the buffer solution. PK b 344 pK b 937 pK a 931 pK b 475 pK a 746.
Weak acid and a half equiv strong base. Buffer solution consists of a mixture of a weak acid and its salt with a strong base. Short way to determine the pH for a buffer.
Strongish acid weak base. Which of the following is a buffer solution. I II and IV d.
The pKb of NH3 is 474. 010 M Ca OH2 and 010 M KOH 010 M LiF and O10 HF O 010 M HC2H3O2 and 010 KF 010 M HBr and 010 M NH4Br none of these are buffer solutions. Has strongish acid strong base.
Which of the following processes will increase the pH of a buffer solution prepared by mixing 089 mol HCOOH formic acid and 076 mol HCOONa sodium formate into a 1-L solution. A solution which resists the change in pH upon the supplementation of an acidic or basic components is known as a buffer. The pH of a buffer solution does not change when the solution is diluted.
B add some HCl into the buffer solution. A buffer solution is formed when appreciable quantities of a weak acid and its conjugate base are mixed together in aqueous solution. It resists the change in p H when small amount of strong acid or base is added to it.
So looking at your list a. I 002 mole of disso asked Dec 22 2021 in Chemistry by Arungupta 242k points. Carbonic acid H 2 CO 3 and sodium bicarbonate NaHCO 3 help buffering human blood because H 2 CO 3 is a weak acid that does not totally dissociate when excess hydrogen ions are present in blood the reaction goes to the left and.
0054 Which of the following solutions has the greater buffer capacity. The following are available as 005 M aqueous solns. A solution which resists the change in hydrogen ion concentration on addition of a small amount of acid or a base in to it.
Weak acid and a half equiv strong base. 1OM KNO3 050M HF 050M NaF 050M HCI none of the options provided is a buffer 1OM NH4Cl 060M NH3. Solution A buffer is a chemical or combination of chemicals that can both take up and release hydrogen ions.
A buffer solution is an aqueous solution of weak acid and its conjugate baseIt can also be a mixture of weak base and its conjugate base. 100 mL of 0200 M HF added to 200 mL of 0200 M NaF. Hair question given is that which of following is a buffer solution so we know there are two types of buffer solution one is acidic and another is basic ok in acidic buffer this is a combination of weak acid salt of salt of weak acid strong base in basic buffer is combination of weak base salt of salt of weak base strong acid ok now we have to find out in these.
Here weak acid is HClO and its conjugate base is NaClO. A buffer solution is prepared by mixing 500 mL of 0300 M NH3 with 50 mL of 0300 NH4Cl. A buffer solution is an aqueous solution consisting of a mixture of a weak acid and its conjugate base or weak base and its conjugate acid.
II III and IV c. Calculate the change in pH of 1 litre buffer solution containing 01 mole each of NH_3 and NH_4CI upon addition of. A buffer is a mixture of a solution a weak acid and its conjugate base or vice versa.
II III and V e. 200 mL of 0400 M HCl added to 100 mL of 0200 M CH3CO2. Which of the following equilibria could be used to support the claim that the addition of a small amount of NaOH to the buffer will result in only a very small change in pH.
The following are the characteristics of buffer. Thus a solution by mixing 100 mL of 0100 M HClO and 50 mL of 0100 M NaOH IS a buffer because. All of the following solutions are buffers EXCEPT a.
Which one of the following pairs of solutions is not an acidic buffer. Which of the following is a buffer solution. 200 mL of 0500 M NaOH added to 200 mL of 1000 M HF.
It is the ability to resist the change in pH on addition of acid or base. Determine whether the mixing of each pair of solutions results in a buffer. 100 mL of 0500 M H2PO4 added to 100 mL of 0200 M HPO42.
Chemistry questions and answers. A solution made by mixing 100 mL of 0100 M HClO and 50 mL of 0100 M HCl IS NOT a buffer. I II IV and V b.
Acid buffer solutions. Acidic buffer are solution of a mixture of weak acid and salt of its. 3D a add some NaCl into the buffer solution.
NH 3 Which solution could be used to prepare a buffer solution with a pH of 450. 200 mL of 0200 M HCl added to 200 mL of 0400 M CH3CO2. CH 3 COOH CH 3 COO H Weakly ionized.
II III IV and V. A buffer solution is made up of acetic acid CH3COOH and sodium acetate NaCH3COO. Buffer solutions have wide applications.
A buffer solution resists changes in its pH when an acid or base is added to it. Use the rearranged K a expressionequation for the weak acid component of the buffer and substitute in moles of HA and A-in place of HA and A- which works because 1 the concentrations of HA and A-in a buffer do not change much as the acid ionization reaction occurs to reach equilibrium because of the common ion effect ie no. The buffer solution is defined as.
Hence solution of acetic acid and sodium acetate is a Buffers solution. The major equilibria in the buffer system are represented above. A buffer acts to resist GROSS changes in pH.
HClO NaOH NaClO. In the given options only option-A has weak acid C H 3 C O O H and its conjugate base C H 3 C O O N a. A buffer is a solution of weak acid and its conjugate base or a weak base and its conjugate acid.
NH3 H2O- NH4 OH- 750 mL of 0125 M HCl is added to the 100 mL of the buffer solution. What is a buffer.

Which Of The Following Is A Buffer Solution Youtube

Chemistry Notes Chemistry Buffer Solution
Buffer Solution Definition Types Uses

Which One Of The Following Is Not A Buffer Solution Youtube

Buffer Solution Acidic Buffer Basic Buffer Animation Chimica

Which Of The Following Is A Buffer Solution

Which Of The Following Is The Buffer Solution Neetlab

Buffer Solution Definition Examples And Preparation

Seven Traits Of Successful Community Managers Infographic Management Infographic Community Manager Social Media Infographic

Top Study World Is One Of The Best Websites In Pakistan Where Students Are Helped In Getting Marks In Ssc Hssc Mcat Vocabulary Pdf Order Of Reaction Syllabus

Which Of The Following Pairs Will Not Form A Buffer Solution Youtube

Which Of The Following Is The Buffer Solution 12 Ionic Equilibrium Chemistry Dinesh P Youtube

What Do You Need To Know To Calculate Ph Buffer Solution Chemistry Chemistry Review

Solubility Common Ion Effect Buffer Solution Buffer Solution Solubility Solutions





Comments
Post a Comment